“Our plan is as clear as it is bold: get more people vaccinated for free, create more places for them to get vaccinated, mobilize more medical teams to get the shots in people’s arms, increase supply and get it out the door as soon as possible.”
– President Biden in a COVID-19 press briefing on Tuesday
“The details still have to be worked out, but this is really a critical recognition that state and local health agencies need to be shored up in a way that they haven’t been in decades.”
– Dr. Michael Osterholm, epidemiologist and director of the Center for Infectious Disease Research and Policy at the University of Minnesota
______________________________________________________________________________________________________
Three subjects consumed financial headlines this week: big tech earnings, a “flash mob” of retail investors that sent an ominous warning to Wall Street via an epic short squeeze, and an announcement by President Biden that COVID-19 vaccine distribution would speed up and reach 300 million Americans by the end of the summer. On the first point, big tech earnings turned out to be mixed, which disappointed markets after one of tech’s biggest companies – based in nearby Redmond, WA – blew analyst predictions out of the water early in the week.
On the second point, a frenzied group of investors on Reddit and Discord pushed GameStop and AMC into the stratosphere through an epic short squeeze aimed at sending a warning shot to Wall Street and bludgeoning activist hedge funds that shorted the securities (see charts below through Wednesday).
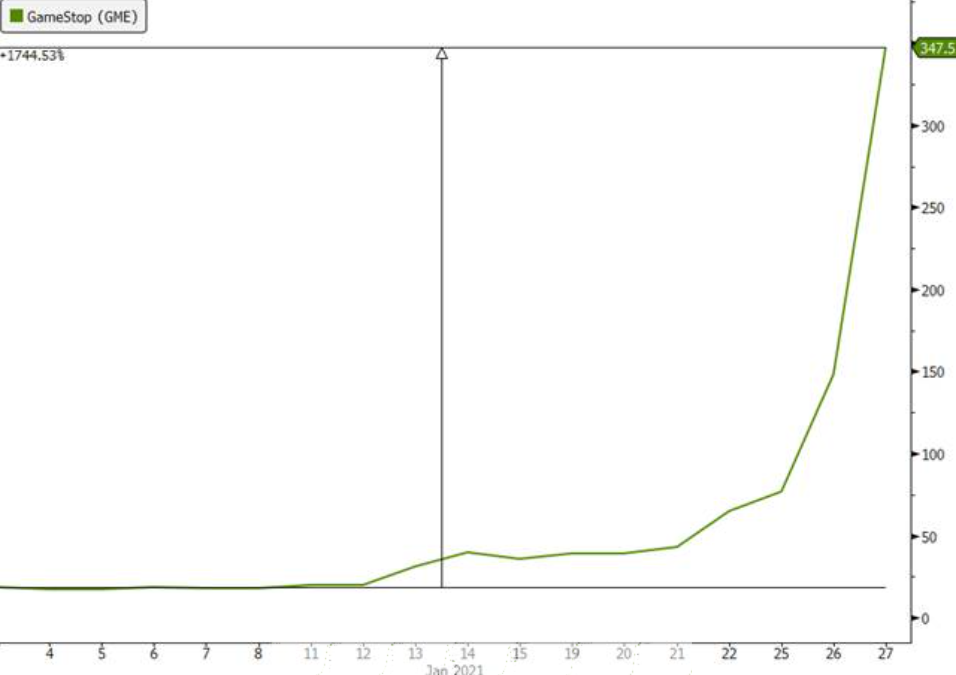
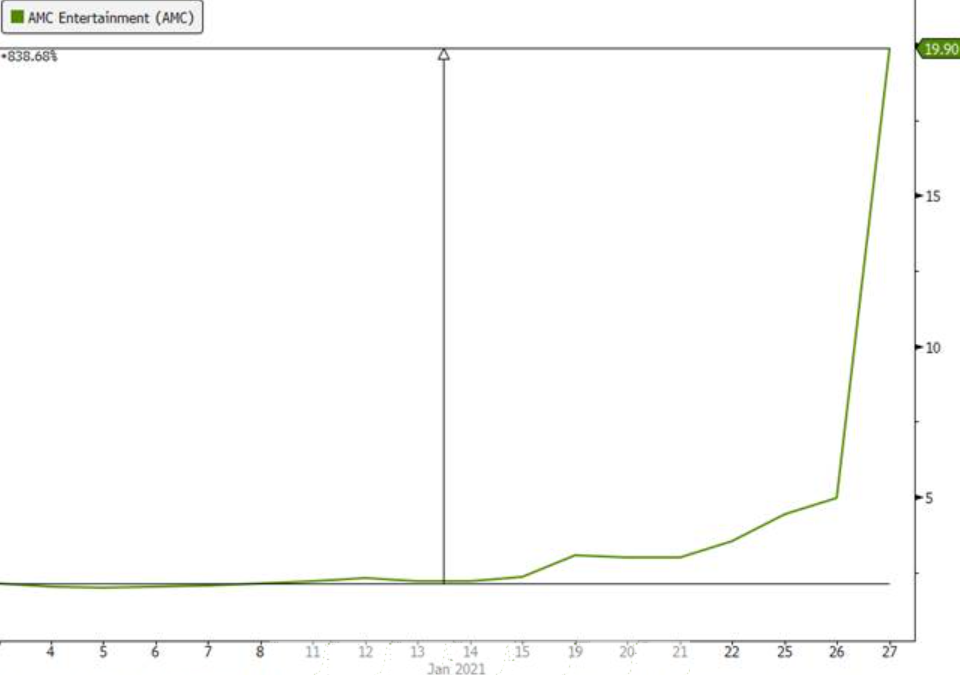
Source: Bloomberg, Evergreen Gavekal
Our esteemed partner Louis-Vincent Gave constructed a compelling narrative regarding the implications for capitalism when retail investor “mobs” organize around specific securities. We will be publishing more about this shocking market event next week, but here is a quick and very telling excerpt from Louis’ piece on the subject:
“The new-found ability of big groups of investors to rapidly organize a ‘flash mob’ means that the possible highs, and lows, for any given listed asset might be multiples of what was seen as possible before. In this regard, bitcoin may be a trailblazer, not because it is set to become a new means of exchange (it isn’t), but for what bitcoin tells us about the potential volatility of assets in our hyper-networked, modern world. This ability to organize market ‘flash mobs’ is as much of a threat to financial systems as the real thing is to retail stores. This effect means that out of nowhere, price discovery for an asset can suddenly become irrelevant. Prices can go anywhere: trillions or zero (or perhaps even below, as we saw with the -US$40 a barrel oil print last April). This is a problem for capitalism.”
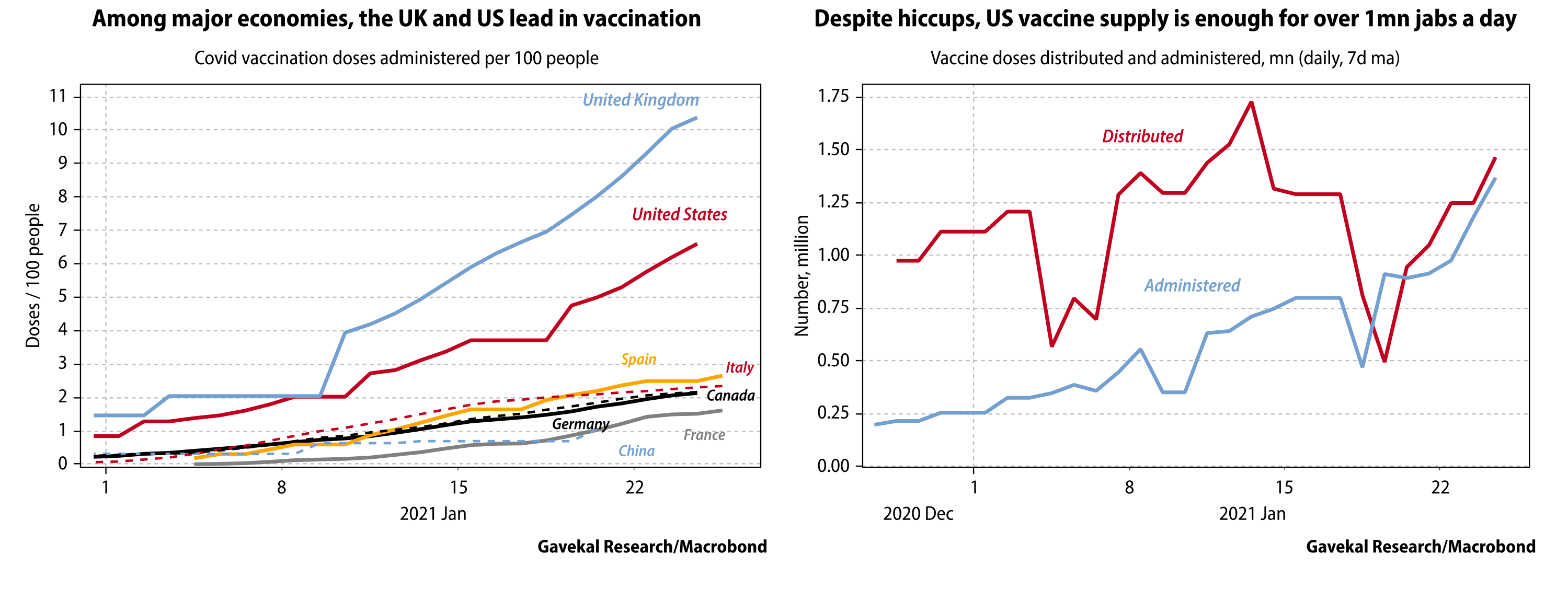
Regarding the third point, in a press conference on Tuesday, President Biden announced that the federal government is working to purchase an additional 200 million doses of Pfizer and Moderna’s COVID-19 vaccines, with the goal of having enough supply to vaccinate the entire adult U.S. population by the end of the summer. With new COVID-19 variants seemingly popping up weekly, the urgency for a rapid and coordinated domestic and international vaccination campaign becomes even more pressing.
In this week’s edition of EVA, we are presenting a pertinent article from one of our colleagues at Gavekal that sheds light on the uphill battle facing the new administration in reaching this goal. Despite the manufacturing challenges around this effort, Dan Wang provides a small glimmer of hope that vaccine manufacturers will learn from early mistakes and correct course. Assuming the vaccination campaign is in fact successful and public confidence is restored over the next several months, we believe the probability of an “economic re-opening boom” is high, as life inches closer to “normal” and consumers release pent-up demand for goods and services.
The race to vaccinate the world in the middle of the Covid-19 pandemic will be one of the most complex projects ever attempted. Governments have approved a dozen vaccines, and manufacturers claim that by the end of 2021 they will churn out doses at a rate of 11bn a year. Most developed economies aim to fully vaccinate their adult population by year’s end.
This target is achievable, but daunting. Most media coverage so far has focused on early distribution hiccups in vaccination campaigns, the possibility that a new highly-transmissible variant of the coronavirus could lead to a new wave of infections before vaccination is widespread, and concerns that another variant first found in South Africa could prove vaccine-resistant.
Yet there is an even more basic worry: that vaccine makers will simply miss their production targets. In 2018, the world produced less than 5bn vaccine doses of all types, including general vaccines, the oral polio vaccine and seasonal flu shots. Now the goal is to produce more than double that amount of just Covid-19 vaccines annually, often using novel technologies, and distribute them under unforgiving conditions. Last week, AstraZeneca reportedly told the European Union that it might deliver only half of the doses that it expected in the first quarter.
Interviews with companies across the vaccine supply chain make clear that such production shortfalls could easily keep happening.
Challenges in manufacturing
The vaccines approved so far employ three main technologies: the traditional cell-culture process using inactivated viruses (the Chinese and Russian vaccines); the viral-vector process in which a harmless piece of coronavirus protein is injected into cells, triggering an immune response (AstraZeneca and Johnson & Johnson), and the messenger-RNA process in which the harmless virus proteins are made by the body’s cells themselves, under instructions from the mRNA in the vaccine (Pfizer/BioNTech and Moderna). Manufacturing processes differ, but all include three basic procedures.
First is the assembly of raw materials. Vaccines use a host of ingredients, ranging from genetic materials and cell cultures to sterile water and specialized chemicals. They require certain rare natural products, such as horseshoe crab blood, which contains a substance used to test for contaminants. Scarcity of raw materials is one of the main risks to the rollout. In interviews, vaccine makers report that they are already seeing long lead times for certain chemicals. Last November, Pfizer announced that it would meet only half of its 2020 production target, in part because it ran out of unspecified raw materials.
The second step is synthesizing the vaccine. Processes vary substantially between vaccine types. Inactivated and viral-vector vaccines are cultured over several weeks in bioreactors which must be in highly-secure Biosafety Level 3 facilities. The cell cultures must then be harvested, cleaned and purified. The mRNA vaccines are quicker to make, through a purely chemical process. But the process for inserting RNA into carrier particles is novel, and has never been done at scale. It is possible that vaccine production could be slowed by problems in these intricate manufacturing processes, despite vaccine makers’ forecasts that they will hit their target production capacities this year. In January, Johnson & Johnson reportedly ran into manufacturing delays that have put its production two months behind schedule.
Third comes the “fill and finish” process that prepares the vaccine for delivery. The vaccine needs to be put into vials, inspected for problems, and packaged for delivery. Much can go wrong. The vaccines have to be tested for safety, efficacy, purity and potency. Manufacturers need specialized glass vials that will not react with the vaccine. Inspection cameras on filling lines have to detect the presence of a vial stopper, the fill level and cracks in the glass, usually at the rate of a few hundred per hour. One production manager has said that current capacity on fill-and-finish inspection lines is far less than needed for a rapid global vaccination campaign.
Once the vaccines are made, tested and packaged, two logistical hurdles remain. One is the cold chain. The mRNA vaccines must be shipped and stored in ultra-cold freezers (-70°C degrees for Pfizer and -20°C for Moderna), with temperature trackers to ensure the vaccine never spoils. Maintaining this cold chain will be a particular risk in developing countries. Finally, abundant supplies of basic medical equipment are needed at the spot where needles enter arms: syringes, swabs, medical gloves and so on. Shortfalls in any of these materials could slow down inoculation drives even if vaccine supplies are ample.
Companies across the vaccine supply chain mostly express confidence that manufacturing bottlenecks can be overcome. But they are keen to underscore that they’ve never had to tackle a project of this scale, and there are many unknown unknowns. It would only take one or two glitches to throw off vaccination schedules by weeks or months. In the early days of the pandemic, for example, the US had trouble producing cotton swabs—by any measure a simple technology—which slowed down its capacity to conduct tests. The US and much of Europe are counting heavily on the Pfizer and Moderna mRNA vaccines, whose production processes have never been scaled up. Moreover, at each step of the manufacturing process, only a handful of vendors produce the specialized equipment, from cell-culture media to glass vials.
The scarcity of vendors is due mostly to high regulatory standards. Vaccines have to be produced in Good Manufacturing Practice facilities, which must meet stringent safety and cleanliness standards. A new GMP plant usually takes from three to five years from groundbreaking to production, mainly because of the need for regulatory approvals. This means that ramping up vaccine capacity is constrained by the number of GMP facilities, and the loss of a manufacturing site to accident or natural disaster cannot easily be made up. Regulators in the US and Europe have started to fast-track new GMP plants, so that they can be up and running in nine to 18 months rather than several years.
This acceleration reduces supply risks but creates other potential problems. If laxer production safety standards lead to vaccines producing more or worse side effects, this could erode public acceptance and slow down the rate of inoculations. Some pharmaceutical executives are still wary of the more innovative Covid-19 vaccines. They know that mRNA technology has never been used to produce a commercial vaccine for humans. That and the significant acceleration in vaccine approval and manufacturing make them nervous.
Three of the major vaccine makers—Pfizer, AstraZeneca, and Johnson & Johnson—have already experienced delays in their production plans. The optimistic scenario is that they will be able to resume their production schedules after retooling their operations and gaining manufacturing experience. But given the sheer number of things that can go wrong, there is good reason to be concerned that the world’s biggest-ever vaccination drive will be slowed by manufacturing and distribution breakdowns.
DISCLOSURE: This material has been prepared or is distributed solely for informational purposes only and is not a solicitation or an offer to buy any security or instrument or to participate in any trading strategy. Any opinions, recommendations, and assumptions included in this presentation are based upon current market conditions, reflect our judgment as of the date of this presentation, and are subject to change. Past performance is no guarantee of future results. All investments involve risk including the loss of principal. All material presented is compiled from sources believed to be reliable, but accuracy cannot be guaranteed and Evergreen makes no representation as to its accuracy or completeness. Securities highlighted or discussed in this communication are mentioned for illustrative purposes only and are not a recommendation for these securities. Evergreen actively manages client portfolios and securities discussed in this communication may or may not be held in such portfolios at any given time.